Submitted by M. Rodrigues on Fri, 28/08/2020 - 11:56
Single-molecule visualization of DNA G-quadruplex formation in live cells
The formation of four-stranded DNA has been tracked in living human cells for the first time,
allowing scientists to see how it works, and its possible role in cancer!
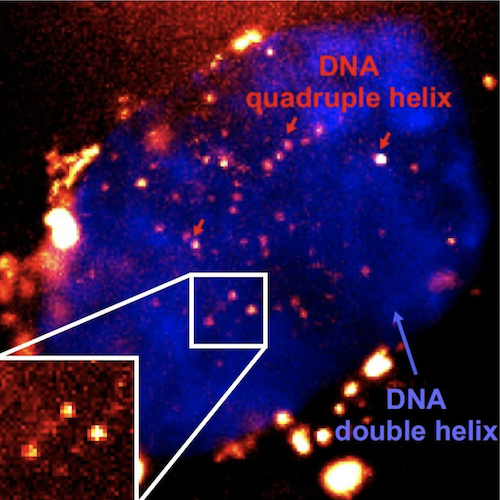
DNA usually forms the classic double helix shape discovered in 1953 – two strands wound around each other. Several other structures have been formed in test tubes, but this does not necessarily mean they form within living cells. Quadruple helix structures, called DNA G-quadruplexes (G4s), have previously been detected in cells. However, the technique used required either killing the cells or using high concentrations of chemical probes to visualise G4 formation, so their actual presence within living cells under normal conditions has not been tracked, until now. A joint research team from the Balasubramanian and Klenerman labs at the University of Cambridge, have invented a fluorescent marker that is able to attach to G4s in living human cells, allowing them to see for the first time how the structure forms and what role it plays in cells. The work recently published in Nature Chemistry, represents a breakthrough in being able to image single G4s and will make it possible to unveil their cellular functions and role in disease.
Rethinking the biology of DNA
Dr Marco Di Antonio, joint first author, now a group leader in the Department of Chemistry at Imperial College London, said: “For the first time, we have been able to prove the quadruple helix DNA exists in our cells as a stable structure created by normal cellular processes. We can track G4s in real time in cells and ask directly what their biological role is.” The team thinks G4s form in DNA in order to temporarily hold it open and facilitate processes like transcription, where the DNA instructions are read and proteins are made. This is a form of ‘gene expression’, where part of the genetic code in the DNA is activated. G4s appear to be associated more often with genes involved in cancer, and are detected in larger numbers within cancer cells. With the ability to now image a single G4 at a time, the team say they could track their role within specific genes and how these express in cancer. This fundamental knowledge could reveal new targets for drugs that interrupt the process.
Live image of single G4s
Dr Aleks Ponjavic, joint first author, now a group leader in the Schools of Physics & Astronomy and Food Science and Nutrition at the University of Leeds, said: “Scientists need special probes to see molecules within living cells, however these probes can sometimes interact with the object we are trying to see. By using single-molecule microscopy, we can observe probes at 1000-fold lower concentrations than previously used. In this case our probe binds to the G4 for just milliseconds without affecting its stability, which allows us to study G4 behaviour in their natural environment without external influence.”
The team’s breakthrough in being able to image single G4s came with a rethink of mechanisms usually used to probe the working of cells. Previously, they had used antibodies and molecules that could bind to the G4s, but these needed very high concentrations of the ‘probe’ molecule. This meant the probe molecules could be disrupting the DNA and actually causing it to form G4s, instead of detecting naturally forming G4s. Now the team used a new ‘bright’ fluorescent molecule designed to stick to the G4s very easily that could be used in small amounts. The small amounts meant they couldn’t image every G4 in a cell, but could instead identify and track single G4s, allowing to understand their fundamental biological role without perturbing their overall prevalence and stability in the cell. Together researchers, were able to show that G4s appear to form and dissipate very quickly, suggesting they only form to perform a certain function, and that potentially if they lasted too long they could be toxic to normal cell processes.
Single-molecule fluorescence imaging of G4s in living cells using the fluorescent probe SiR-PyPDS
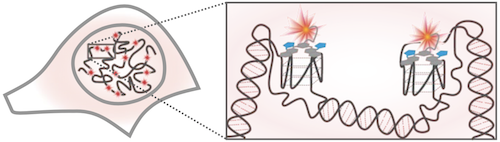
Schematic of G4s in the cell nucleus, with a magnified view showing G4s stained by SiR-PyPDS
Reference: Di Antonio, M., Ponjavic, A., Radzevičius, A. et al. "Single-molecule visualization of DNA G-quadruplex formation in live cells", Nat. Chem. 12, 832–837 (2020). https://doi.org/10.1038/s41557-020-0506-4