Submitted by M. Rodrigues on Mon, 02/11/2020 - 17:38
Abscission couples cell division to embryonic stem cell fate
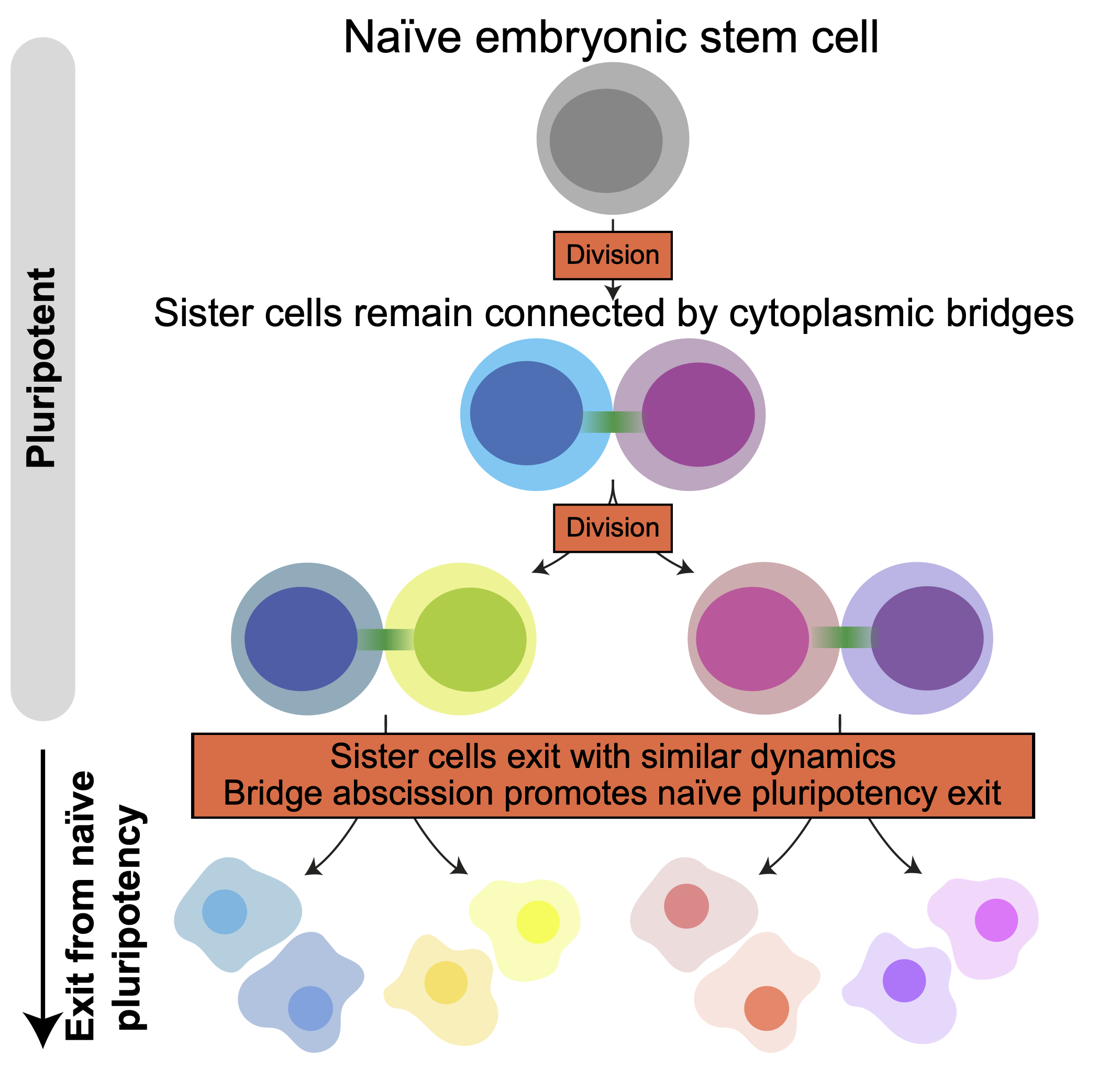
In the early embryo, cells change fate from a pluripotent state where they have the potential to become any cell type, towards more and more specialised cell types. One such fate transition is exit from naive pluripotency, when cells become receptive to differentiation signals; at the same time, cells rapidly divide to proliferate and increase the size of the embryo. In stem cells extracted from the mouse embryo, cell division and naïve pluripotency exit are coupled because inhibiting cell division impairs fate transition; yet the underlying mechanisms are unknown.
In this study, recently published in Developmental Cell, the Paluch Lab investigated the coupling between cell division and exit from naïve pluripotency. Using mouse embryonic stem cells, they demonstrated that cells exit pluripotency directly after cell division, and that exit dynamics are tightly coupled between daughter cells, even when the two cells have considerably different sizes. This coupling results from the fact that when a naïve stem cell divides, the two daughter cells remain connected by a bridge for many hours after cell division, effectively still exchanging material even though division took place. Severing of this bridge accelerates during exit from pluripotency. Interestingly, artificially maintaining the bridges delays exit from naive pluripotency, while cutting them precociously using a laser accelerates exit. “I think the most exciting finding of the paper is that cell division can be modulated to allow cell fate transitions. Before, we imagined cell division as a mechanism that only serves for increasing the number of cells in the organism. Now we know that it can be crucial to allow cell fate transitions, and that in turn that fate transitions can change the way cell divide, and in particular modulate the rate of abscission. Cell division is actually much more dynamic than we thought during development!”- said Dr. Agathe Chaigne, first author of the study. This works sheds light on how a basic process, such as cell division, can be remodelled quickly during development to allow transitions between fates.
Abscission increases when cells exit naive pluripotency
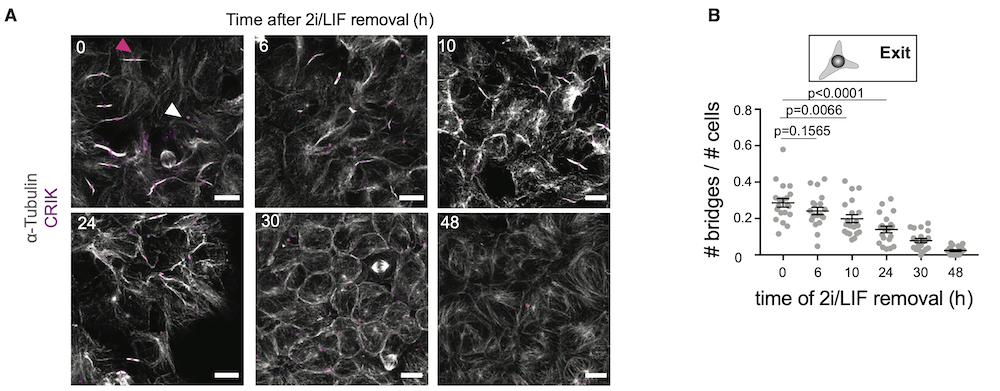
(A) Representative confocal images of cells at different stages of naive pluripotency exit and stained for a-tubulin (white) and CRIK (magenta). Pink arrowhead, example of a bridge with a CRIK spot; white arrowhead, example an isolated CRIK spot, suggesting a midbody remnant. Cells are cultured on laminin to facilitate the visualization of the bridges. Scale bars: 10 mm.
(B) Dot plot showing the fraction of cells with bridges (number of bridges divided by number of cells in a given analysis frame) in H2B-RFP ES cells and during naive pluripotency exit on laminin. Mean and standard error of the mean are shown. N = 2.
Reference: Chaigne A, Labouesse C, White IJ, Agnew M, Hannezo E, Chalut KJ, and Paluch EK. Abscission couples cell division to embryonic stem cell fate. Developmental Cell 55, 1–14 October 26, 2020. https://doi.org/10.1016/j.devcel.2020.09.001