Submitted by M. Rodrigues on Mon, 29/06/2020 - 11:47
Cortical cell stiffness is independent of substrate mechanics
Over the past decade, cell mechanics measurements have repeatedly shown that cells growing on soft materials become soft, and those growing on stiff materials become stiff. This observation has held true for many different cell types, including stem cells and cancer cells. This idea of ‘mechanical adaption’, whereby cellular properties adjust to the physical properties of the surrounding material, has therefore become a largely unquestioned presumption in mechanobiology. Research from the University of Cambridge has now demonstrated that this may not be entirely true.
The method, reported in the journal Nature Materials, will enable a more accurate measurement of cell mechanics within biological tissues and has overturned a long-held dogma in mechanobiology.
“Initially, we only wanted to confirm if cells of the nervous system also stiffen on stiffer substrates, as it had been shown for many other cell types before.” said Dr. Johannes Rheinländer, first author of the study. “And they did. However, when we took a closer look, we realized that our results might have been affected by a mistake in how these experiments are generally analysed.”
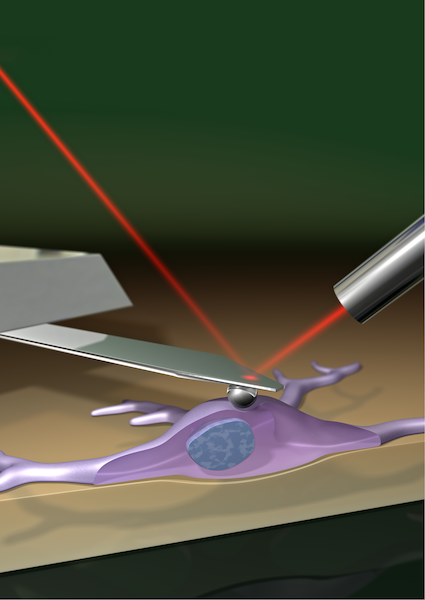
Cell stiffness is frequently measured using atomic force microscopy (AFM), a tiny cantilever is used to press on a cell, and calculated by combining how much the cell is deformed with the applied force. “When a cell sits on a soft material, the applied force does not only deform the cell, but it also pushes the cell into the underlying material, in the same way as when my kids jump on me when they wake me up in the morning, they don’t just squish me but also push me into the mattress.” said the study’s lead author Dr. Kristian Franze, from Cambridge’s Department of Physiology, Development and Neuroscience. “And if this deformation of the underlying substrate is not taken into account – and it usually isn’t – cells on soft substrates will appear softer than they actually are!”
Together with the groups of Prof. Guillaume Charras from University College London, Prof. Malte Gather from the University of St. Andrews, and Dr. Kevin Chalut from the University of Cambridge, the team examined this effect carefully and confirmed that when they used an AFM to press on cells growing on soft materials, the materials themselves were indeed deformed. Next, soft elastic beads as small as the cells, fabricated by the group of Prof. Timo Betz from the University of Münster, were placed on soft and stiff substrates. The results were striking, the data suggested that beads were softer on soft substrates and stiffer on stiff substrates, an unfeasible scenario considering that bead stiffness was constant and impossible to alter.
To resolve this dilemma, the team developed a new, straightforward way to analyse AFM data which considered the deformation of the soft surroundings. When this new method was applied, the effect of the underlying material on the bead stiffness disappeared: “We now find that the beads have identical stiffnesses on soft and stiff environments, which makes much more sense.” said Rheinländer.
Armed with this new method, the team again checked the living cells. Surprisingly, the stiffness of the cells no longer adapted to the stiffness of their surroundings: like the beads, they maintained the same stiffness whether they were grown on a soft or a stiff material. More studies are needed to confirm that cells generally do not adapt their own stiffness to that of their environment. Nonetheless, this works call into question a long-standing axiom in cell mechanobiology.
Numerous recent breakthroughs have shown that mechanical factors are important in many biological processes, such as tissue morphogenesis, stem cell biology, wound healing, and cancer development. The lack of mechanical adaptation of cells may, for example, play an important role in disease processes like cancer metastasis, in which soft cancer cells squeeze through tough tissues. “We are only beginning to understand how mechanical signals impact cells in health and disease,” says Franze, whose team focuses on the mechanobiology of the nervous system. “There are many fascinating questions waiting to be answered in the future, and new tools and approaches will help answer these questions.”
Reference: Rheinlaender, J., Dimitracopoulos, A., Wallmeyer, B. et al. Cortical cell stiffness is independent of substrate mechanics. Nat. Mater. (2020). https://doi.org/10.1038/s41563-020-0684-x