Anna Lippert
Molecular mechanisms of lymphocyte activation - please mind the gap
Keywords: biophysics, mechanobiology, immunology, single molecule imaging
In our body, immune cells, so called lymphocytes, relentlessly rid us of abnormal, malignant or infected cells. However, the initial steps of triggering this immune response are still not understood. On the surface of immune cells are numerous different proteins, some of which are responsible for recognizing inflicted cells, others for initiating the immune response upon recognition, and others still in charge of stopping the triggering.
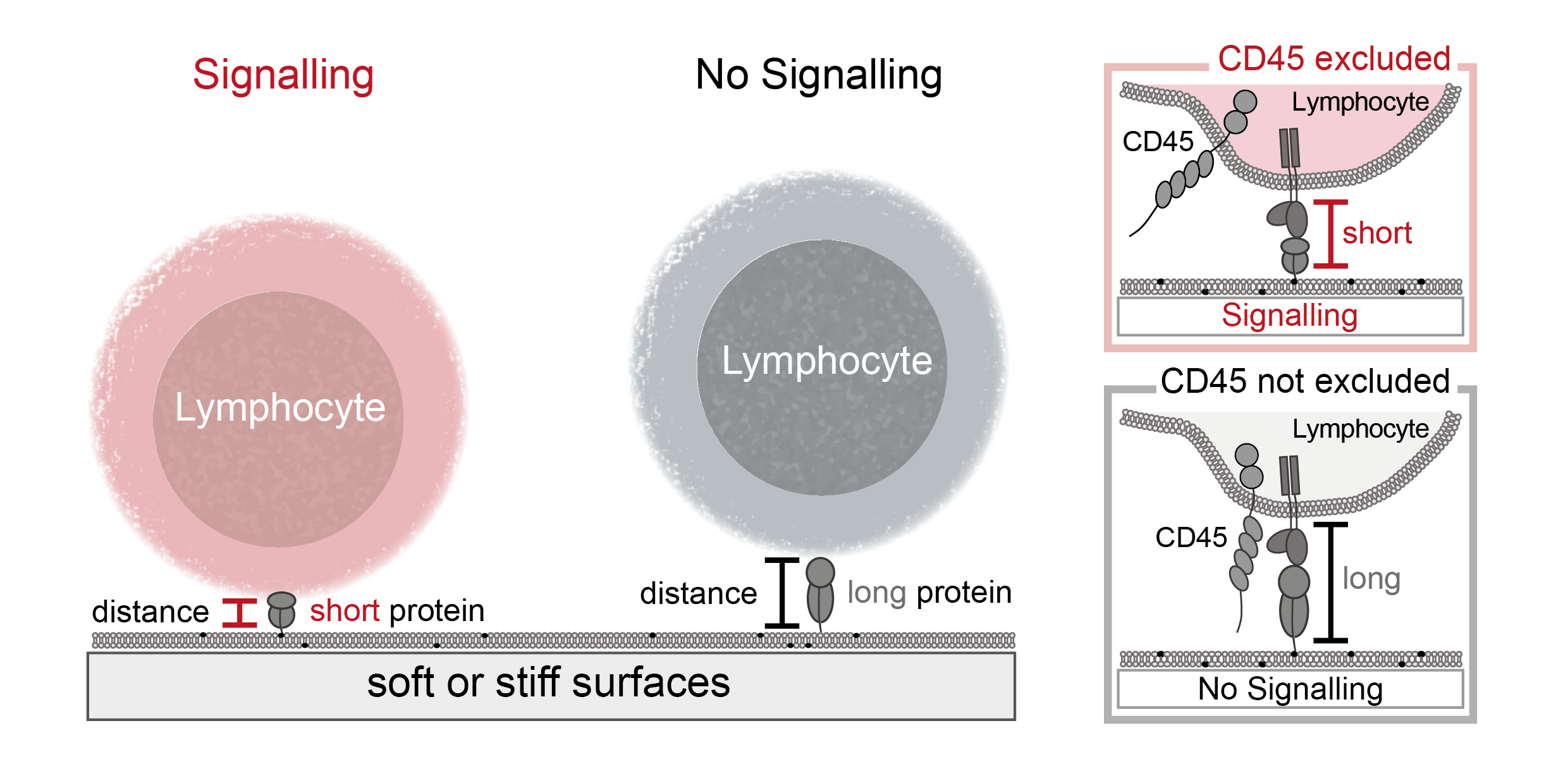
Interestingly, one of the important deactivating proteins, called CD45, is around 22 nm high, which is almost twice the distance between a target cell and an immune cell (around 14 nm). This observation inspired the kinetic-segregation model, which postulates that a contact formed between a target and an immune cell would exclude these large, deactivating proteins, while the smaller activating proteins remain and can initiate triggering. This model has some interesting consequences, most notably that this distance, crucial to shifting the balance of activating and deactivating proteins, could control the immune response. If this model holds true one can manipulate the immune response by solely influencing the distance between immune cells and target cells, having impact on not only activating but also deactivating the immune response. This is especially important for autoimmune diseases, where there is an exacerbate activation of the immune response and not many therapeutic interventions are available.
Using single-molecule and bulk fluorescence microscopy, I tested the kinetic-segregation model by using different proteins to change the distance between the immune cells and a surface while surveying the immune cell activation and CD45 exclusion. My work demonstrated that antibodies from clinical trials influence the distance between immune cells and a surface and thereby the exclusion of CD45 and that varying these distances can cause triggering of the immune response. In the second part of my project I found that the principle of distance induced activation holds true for different cells of the immune system as well. In the last part of my PhD I focused on the phenomenon that stiffer surfaces lead to an increased immune response compared to softer surfaces. Using substrates of different stiffnesses, we showed that kinetic-segregation occurs even on soft surfaces.
My work has demonstrated the importance of the kinetic-segregation model for immune cell triggering and its concepts are at the final stage towards patenting and commercialisation.
Anna's ORCID 0000-0002-0771-8491 and twitter @AnnaHelenaLipp1
Klenerman Lab - Department of Chemistry