Eva Kreysing
Effects of substrate stiffness on membrane tension
Keywords: membrane tension, substrate stiffness, optical tweezers, fibroblasts, neurons
Animal cells possess an outer lipid membrane, which forms a diffusional barrier to the cell’s environment. This cell membrane is closely linked to an underlying layer of the cell’s skeleton, the actin cortex. Both actin cortex and cell membrane are under tension. Cortical and membrane tension regulate many cellular processes such as cell migration, division, stem cell fate choice and endocytosis. However, while we know that the tension of the actin cortex is regulated by substrate stiffness, and the underlying molecular pathways are largely understood, if and how cortical and membrane tension are linked is still poorly understood, and if membrane tension is regulated by substrate mechanics is unknown.
A possible explanation for this lack of data is that membrane tension is difficult to measure. First, the plasma membrane and the underlying actin cortex form a highly entangled structure. Using optical tweezers (OT), we can attach a sticky bead to the membrane, and measure the force required to pull a string of membrane – a tether – away from the cell. This force increases with increasing membrane tension. Using this technique, we determined (i) the peak force (PF), which is required to separate the membrane from the underlying cortex, and (ii) the steady state force (SSF), which is measured when holding the bead in a fixed position. The SSF is thought to scale with membrane tension.
To test the effect of substrate mechanics on membrane tension, we cultured two cell types with fundamentally different cortical structures, neurons (nerve cells) and fibroblasts (connective tissue cells), on glass and hydrogels of different stiffnesses. We observed much higher PFs in fibroblasts on soft hydrogels compared to glass. In contrast, in neuronal axons, which lack an actin cortex, no differences in PFs were observed between glass and soft hydrogels, suggesting that PF can be used as a readout for the coupling between the membrane and the underlying actin cortex.
Additionally, we observed higher SSFs on hydrogels compared to glass in both cell types but no differences between hydrogels of different stiffness. We correlated these results with measurements of forces the cells applied to the substrate through their contractile actin cytoskeleton.
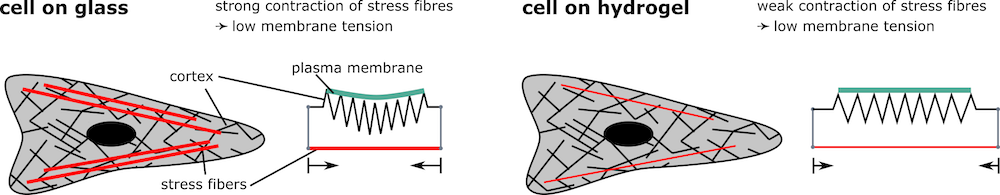
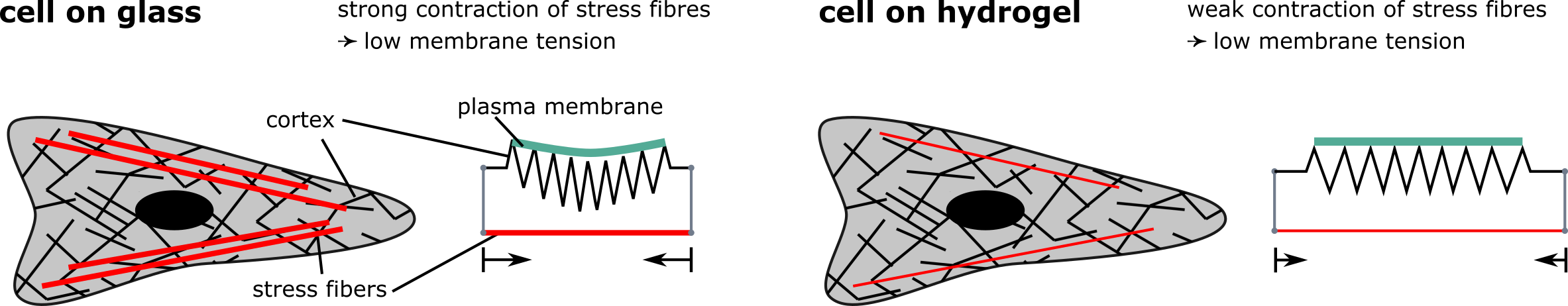
In agreement with previous studies, fibroblasts applied higher forces on stiffer substrates. However, membrane tension was lower on glass, suggesting that increased tension along actin fibres decreases tension in the membrane. A similar effect seems to occur in neurons, yet the explanation might be slightly different. Here the growing tip of the axon pulls harder on the axon if it is grown on stiffer substrates, this way reducing the plasma membrane tension.
Our results show, for the first time, how membrane tension changes in response to substrate mechanics. Combining expertise and technology available at PDN and the Physics department, this new collaboration led to a new model relating cortical tension and membrane tension, explaining our surprising results.
Department of Physiology, Development and Neuroscience
Franze Lab - @Franze_Lab
Figure legend: Toy model to explain observed differences in membrane tension in fibroblast grown of different substrates. Fibroblasts grown on glass exhibit strong stress fibres. This might result in a stronger contraction which decreases the tension in the surrounding cortex and the cell membrane. Fibroblasts on soft substrates exhibit comparatively few and fine stress fibres which might not reduce the tension in the cortex and the membrane as much as in the cells on glass. This could explain why the membrane tension in cells on hydrogels is higher compared to cells grown on glass.