Submitted by M. Rodrigues on Wed, 29/09/2021 - 09:32
Aurora B-dependent polarization of the cortical actomyosin network during mitotic exit
The work recently published by the group of Prof. Buzz Baum (LMB) in EMBO reports explores a novel role of Aurora B kinase in cell division.
Background
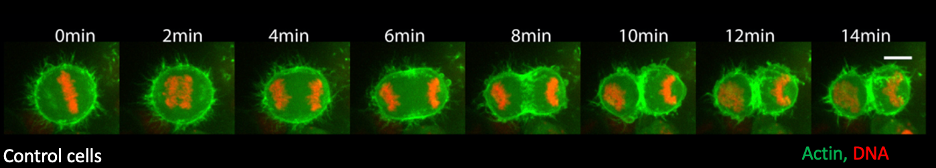
Cell division involves the segregation of genetic material and cellular components into two daughter cells. Cytokinesis is the final step in this process, where an actomyosin ring constricts to cleave the cell into two. Most eukaryotic cells adopt a spherical shape as they enter mitosis, facilitated by the formation of a uniform cortical actomyosin network.
The actomyosin network is progressively patterned during mitotic exit as the cells divide to give rise to two daughter cells. This polarization is driven by two processes happening simultaneously (see below), making it difficult to define their regulation:
- The cells form a contractile actomyosin ring in the center, which constricts and bisects the cell. This has been extensively studied and we now know a battery of players regulating actomyosin ring formation.
- The elongating anaphase spindle causes relaxation of the polar actomyosin network, setting up a gradient in cortical contractility. This process is relatively underexplored, and we are yet to understand how the two processes work together during cytokinesis.
Aim of the project
The goal of the project was to identify novel players regulating relaxation of polar actomyosin network and determine how they cooperate with pathways involved in contractile ring assembly during mitotic exit.
To this end, the authors used high resolution live cell imaging to visualize this process and flat monopolar cytokinesis to artificially bring DNA close to the cortical actomyosin network during anaphase, to directly test the ability of the players to relax actomyosin network.
Key findings
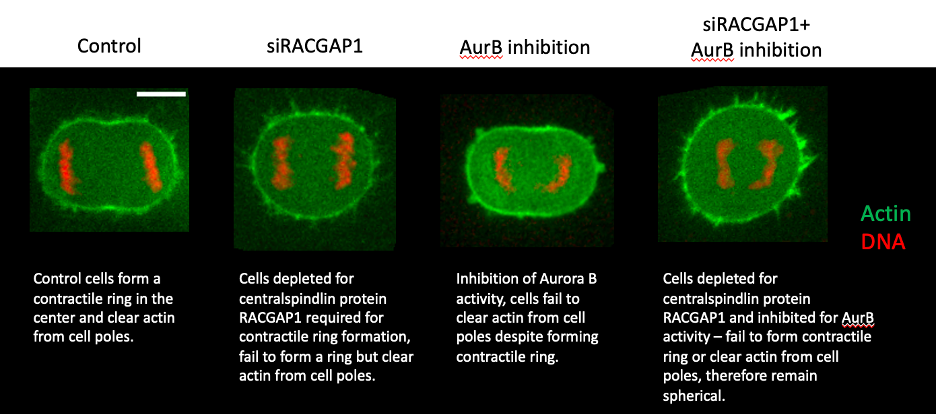
“One of the key findings was the observation that cells can lose actin from their poles during anaphase (when chromosomes separate), despite being unable to form a contractile ring, suggesting that although temporally coupled, the two processes (A and B) are independent of each other”, explains Dr Ramkumar lead author of the paper.
Examining the signals regulating this polar clearance, the authors uncovered a novel role for Aurora B kinase, a core member of the chromosomal passenger complex (CPC), implicated in multiple processes during mitosis. Importantly, the authors found that upon inhibition of Aurora B activity, cells are unable to clear actin from their poles during both monopolar and bipolar mitotic exit. Additionally, using their flat monopolar assay, they found that inhibition of Aurora B activity, directly affected the ability of DNA to clear the underlying actomyosin network.
To further disentangle the contribution of polar relaxation and contractile ring assembly during cytokinesis, the authors inhibited the pathways A and B separately or together – in two different cell lines. Consistently, they found that while individually they had moderate effects on cell shape changes during anaphase, when combined, they had a stronger effect and cells completely failed to change shape, suggesting that the two pathways function parallelly during cytokinesis.
Impact and Implications
The authors show that Aurora B facilitates DNA-mediated polar relaxation and functions to couple the anaphase spindle with the actomyosin cytoskeleton for robust cell division. While much remains to be explored about the mechanism and conservation of its function across systems, Aurora B effectively orchestrates and coordinates multiple events for a successful cytokinesis.
It would be crucial to identify downstream targets of Aurora B, as it could either directly phosphorylate and regulate the activities of cytoskeletal modifiers or could work indirectly through PP1 phosphatase, a known regulator of polar relaxation.
Additionally, the authors demonstrate that the formation of contractile ring and polar relaxation are spatially distinct processes, working parallelly and could be independently regulated during cytokinesis. It would be interesting to investigate how these signals are tailored based on cell type and cellular context. For example – epithelial vs mesenchymal cells, cells dividing in tissue. Particularly, it would be exciting to explore how these pathways are adapted in cells undergoing size asymmetric division.
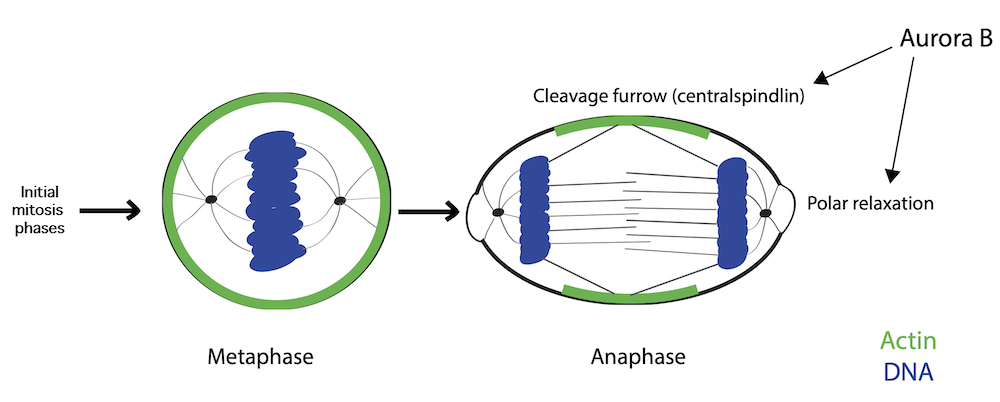
Graphical abstract
Proposed mechanism of action for protein Aurora B
Figure legend: As cells exit mitosis, the uniform actomyosin network is gradually patterned for successful cell division. The protein Aurora B facilitates DNA-mediated relaxation of the polar actomyosin network, thereby, polarizing the actomyosin network. In doing so, it couples the elongating mitotic spindle with changes to the actomyosin network and helps facilitate cell shape changes during mitotic exit.