Submitted by M. Rodrigues on Thu, 08/04/2021 - 21:43
Time-resolved single-cell analysis of Brca1 associated mammary tumourigenesis reveals aberrant differentiation of luminal progenitors
The work recently published in Nat. Commun. offers an explanation as to why the breast is particularly susceptible to tumour development in women with pathologic variants of BRCA1 and reveals a new target for early detection and intervention for these women.
This study is a collaboration between a breast cancer research group led by Dr. Walid Khaled (University of Cambridge) and a statistical genomics lab led by Dr. John Marioni (EMBL-EBI, CRUK-CI) with support from two clinical labs, the Caldas group (University of Cambridge) and the Clarke lab (University of Manchester).
One of the major hurdles for the early detection of cancer is our poor understanding of tumour-initiating events. Historically, cancer research has focused on histological and molecular characterisation of established tumours, which has led to the identification of hundreds of putative mutations that drive tumour development.
“Despite advances, it is still unclear how these genetic aberrations in tumour-initiating cells impact the cell state of nascent tumour cells and how the surrounding cells react” says Dr Bach, co-first author of this paper.
"It is known that loss of function (LOF) mutations of the tumour suppressor gene BRCA1 drive the development of triple negative breast cancer (TNBC) which starts in a cell type called luminal progenitor cell. However, it remains unknown why BRCA1 loss in this cell type leads to the formation of tumours", explains Dr Pensa, also co-first author of this paper.
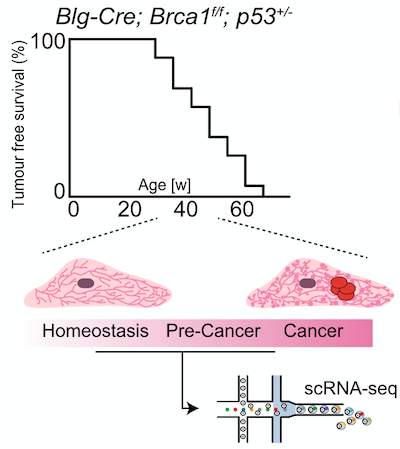
To address this question, a team of researchers led by Dr. Bach and Dr. Pensa conducted single cell RNA-sequencing (scRNA-seq) of mammary gland tissue from a mouse model that harbours a LOF of BRCA1 in luminal progenitors. They collected morphologically healthy tissue at different time points before tumours developed and then were able to computationally reconstruct the tumour development process.
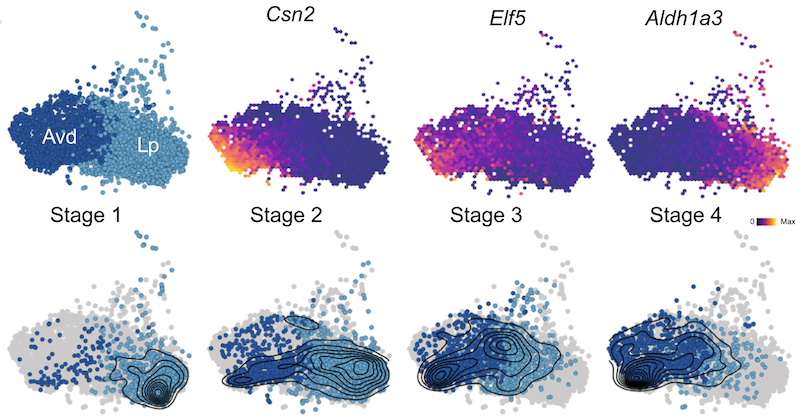
One of the most striking results was to find that during tumourigenesis, a cell type referred to as alveolar cells, expands! In normal physiology, alveolar cells produce and secrete the constituents of milk during pregnancy and lactation. Thus, they only exist when induced by the presence of pregnancy hormones. However, the authors found that upon the loss-of-function of BRCA1, luminal progenitor cells aberrantly give rise (i.e. differentiate) to these cells. Data suggests that the genetic mutation induces a signalling process that is hardwired in luminal progenitors to produce large amounts of the alveolar cells. “We found that the appearance of this cell type is accompanied by changes in the surrounding immune cells. In particular we found several signs of the surrounding immune compartment becoming permissive to the development of tumours.”, reveals Dr Bach.
Importantly, the aberrant differentiation process identified in this work offers an explanation as to why the breast is particularly susceptible to tumour development in women with pathologic variants of BRCA1. Moreover, the study also revealed a new target for early detection and intervention.
Using this new technology and having characterized the earliest aberrant cell state in the gland will facilitate the development of ways to monitor changes in women that are high risk of developing breast cancer. The possibility to accurately monitor tumour development is crucial for clinical decisions and represents a promising help for women carrying the mutation.
Figure legend: Schematic overview of the experimental design. Mammary glands from 13 animals between 30 and 48 weeks of age as well as two fully developed tumours were prepared for scRNA sequencing after depleting dead cells.
Figure legend: The top row highlights the location of the two clusters (Lp and Avd) as well as gene expression of three marker genes. The bottom row is facetted by stages with overlaid density estimate.
Reference: Bach, K., Pensa, S. et al. “Time-resolved single-cell analysis of Brca1 associated mammary tumourigenesis reveals aberrant differentiation of luminal progenitors”. Nat. Commun. 12, 1502 (2021). https://doi.org/10.1038/s41467-021-21783-3